Co-developed
The tool was co-developed with a large community of stakeholders (more than 100 people from 51 organisations), representing patient associations, industry, academics, researchers and external experts.
Simple
Who can use the tool? Simple. All stakeholders working on patient engagement projects (for example, patient advocates, clinical investigators, researchers, or sponsors).
Suitable for all projects
You can use it for a patient engagement project that takes place at any point along the medicine and medical device development and can be applied to health and social research.
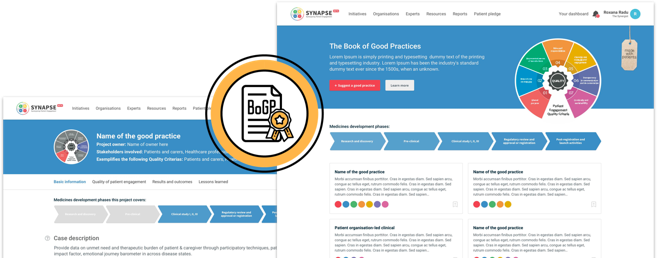
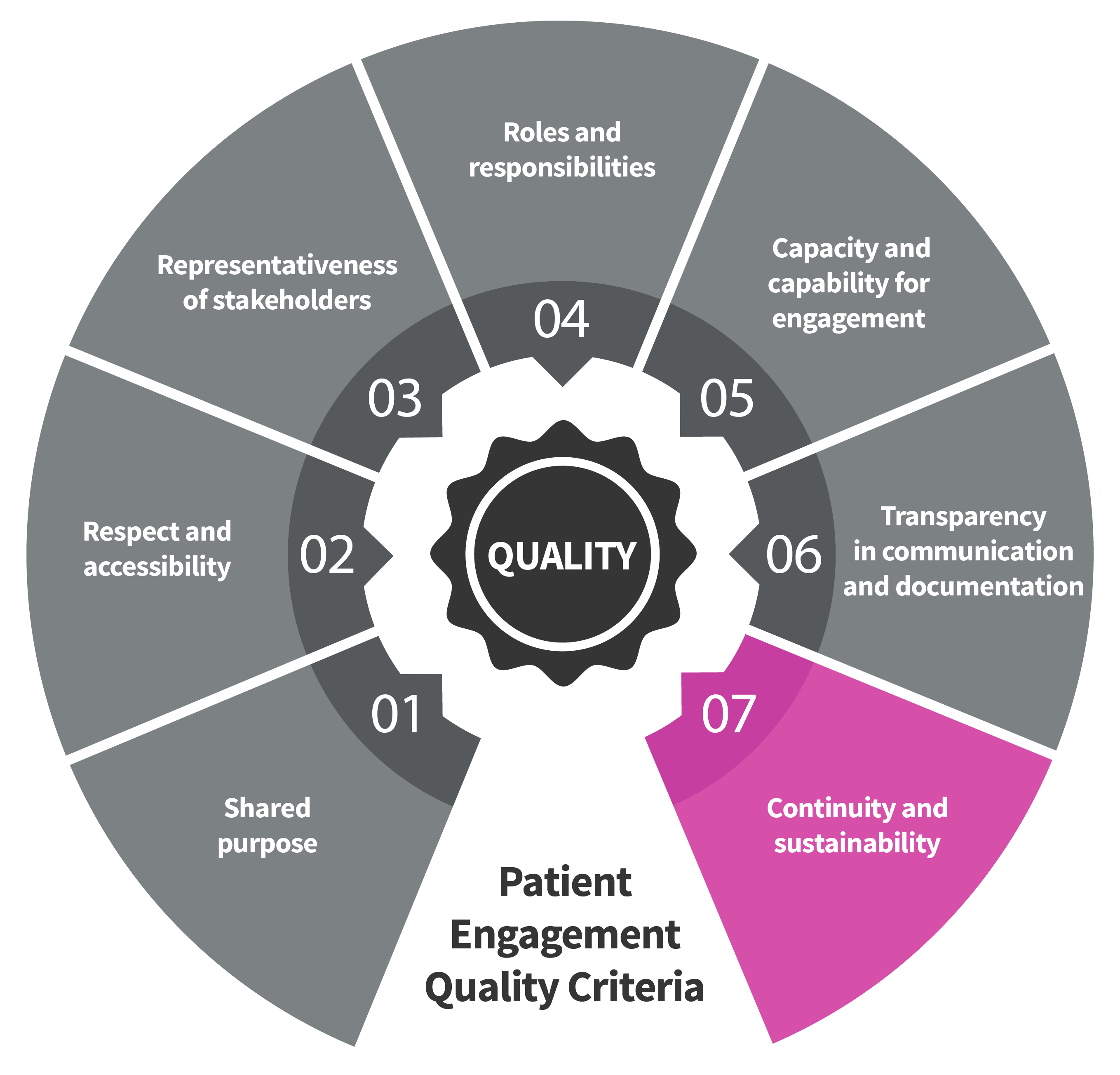
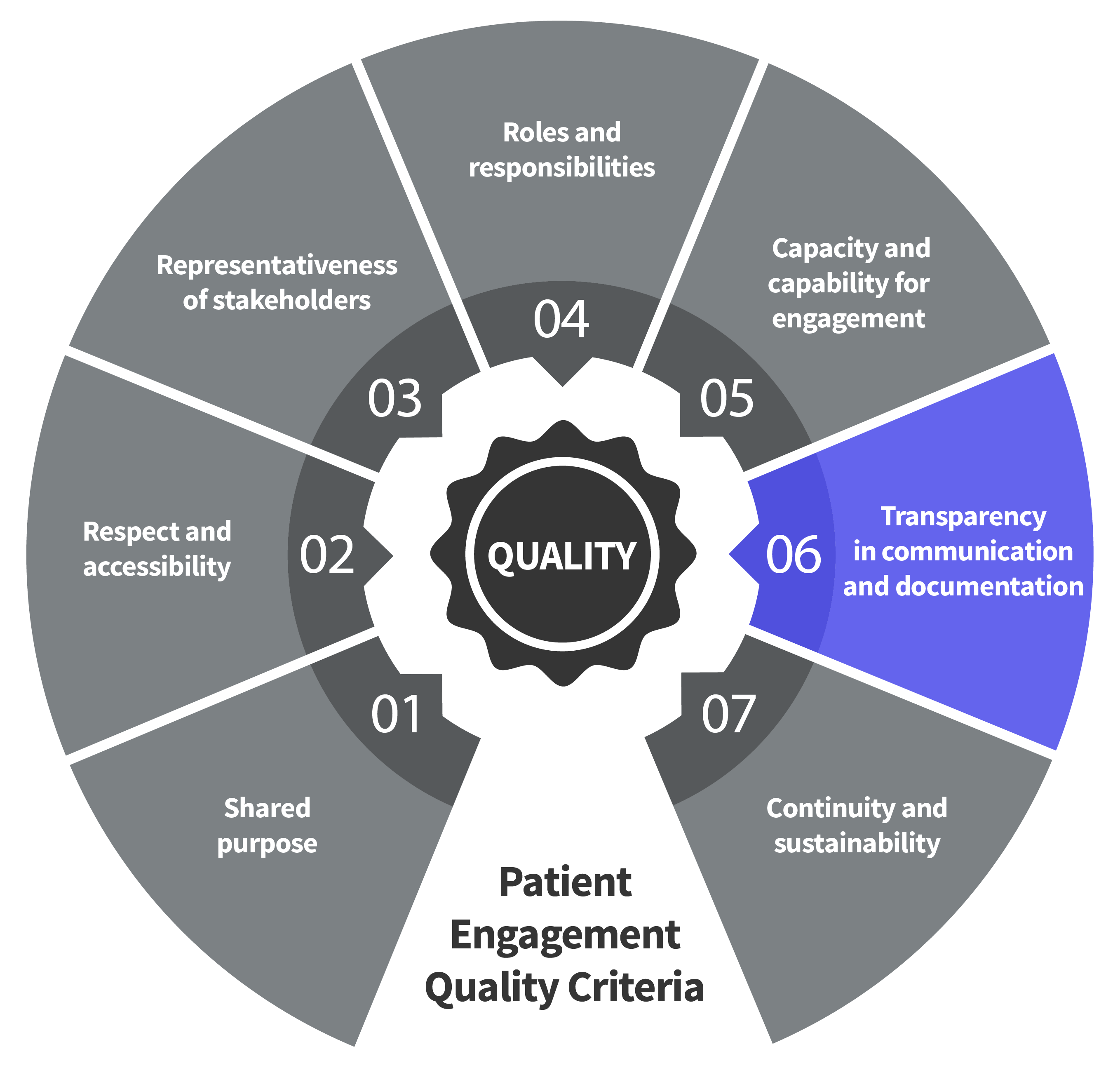
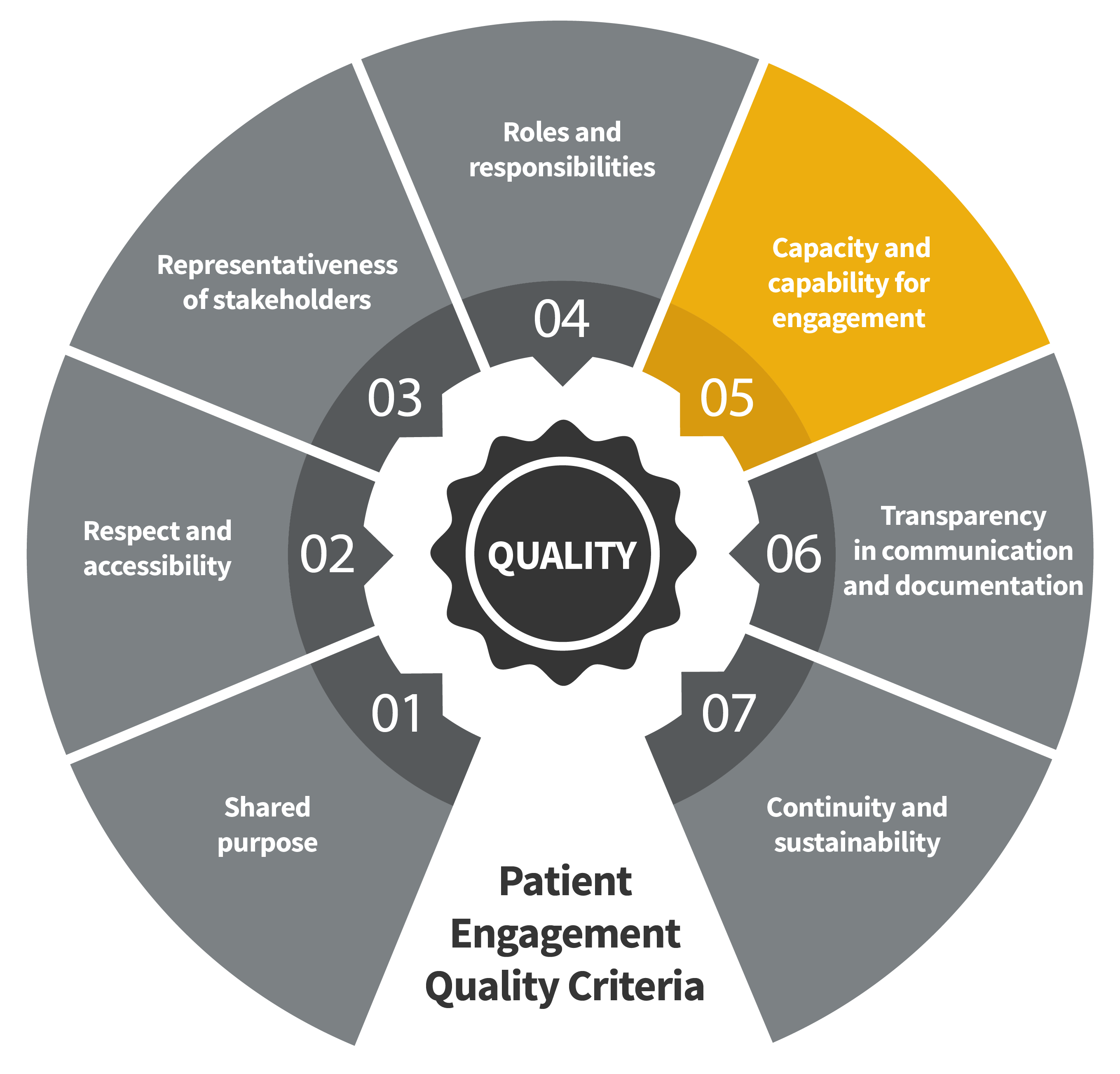
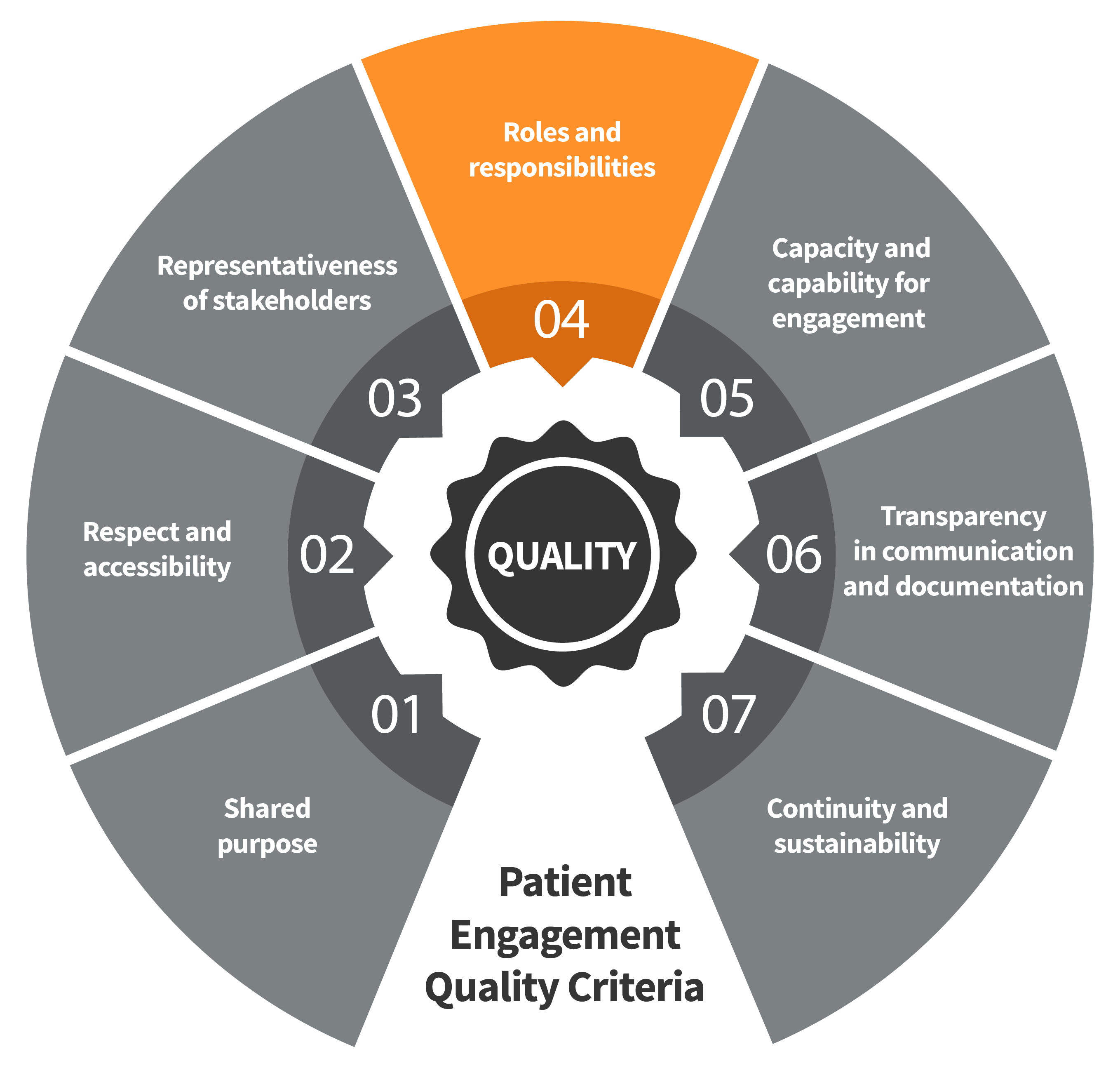
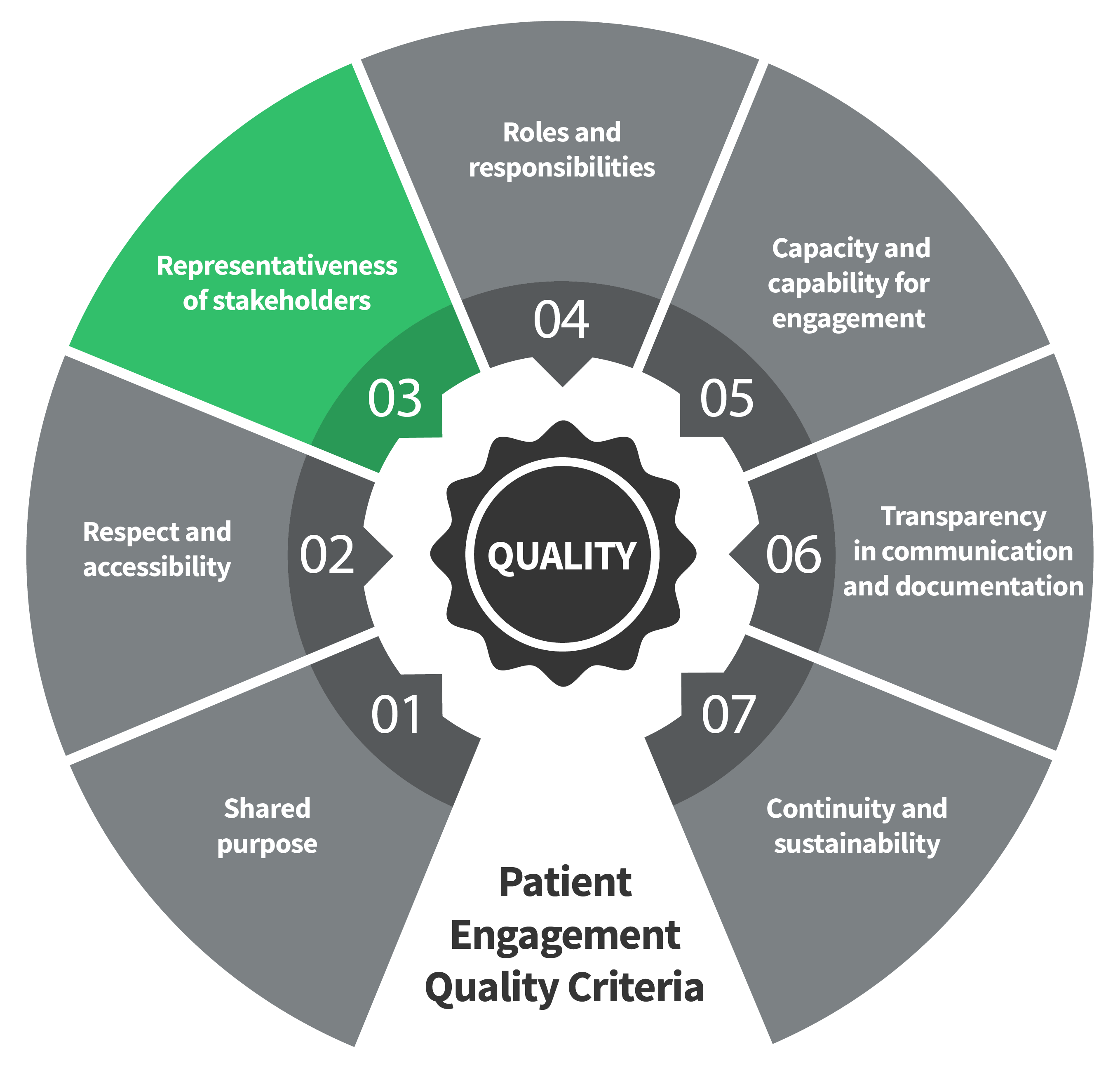
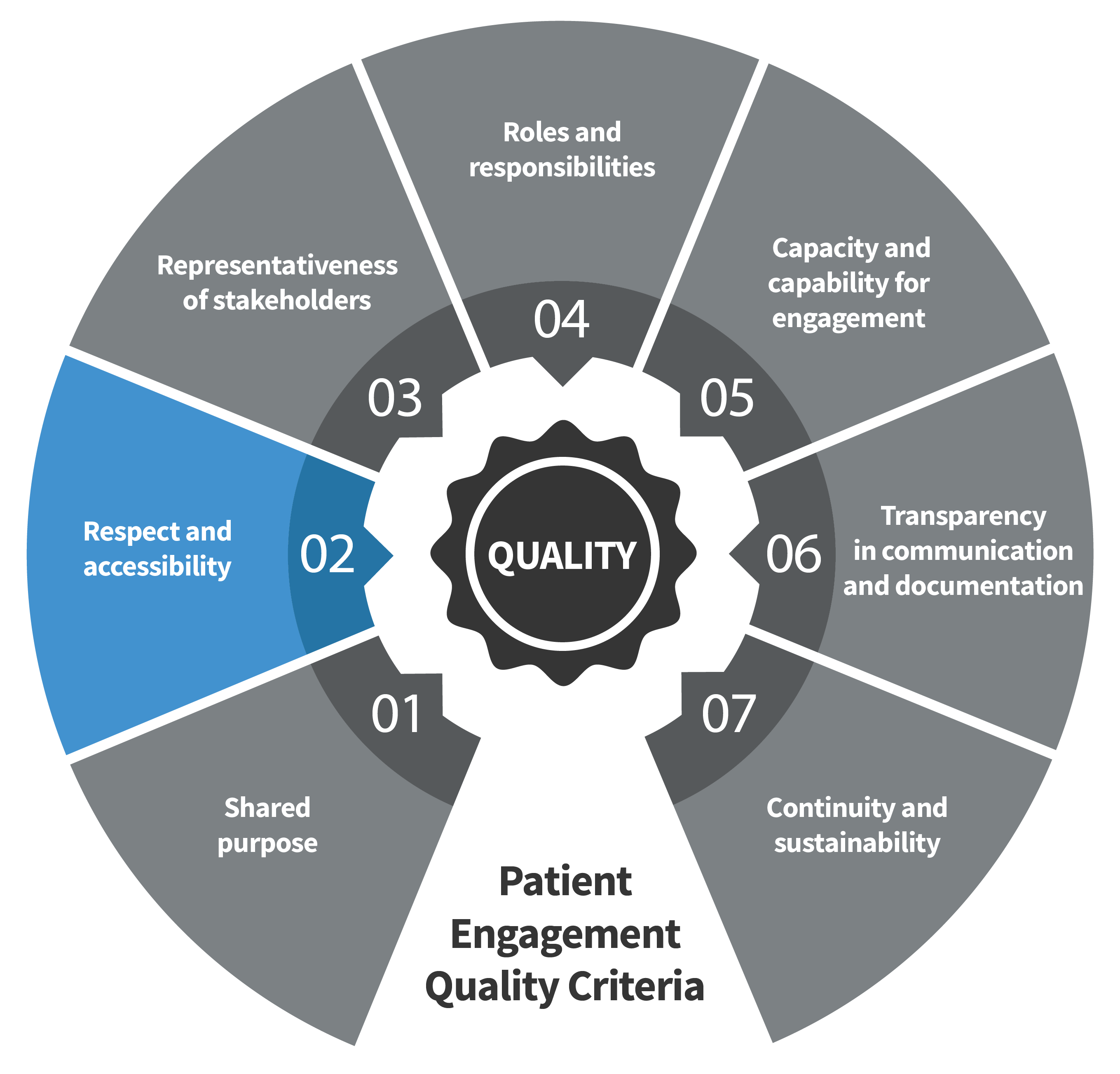
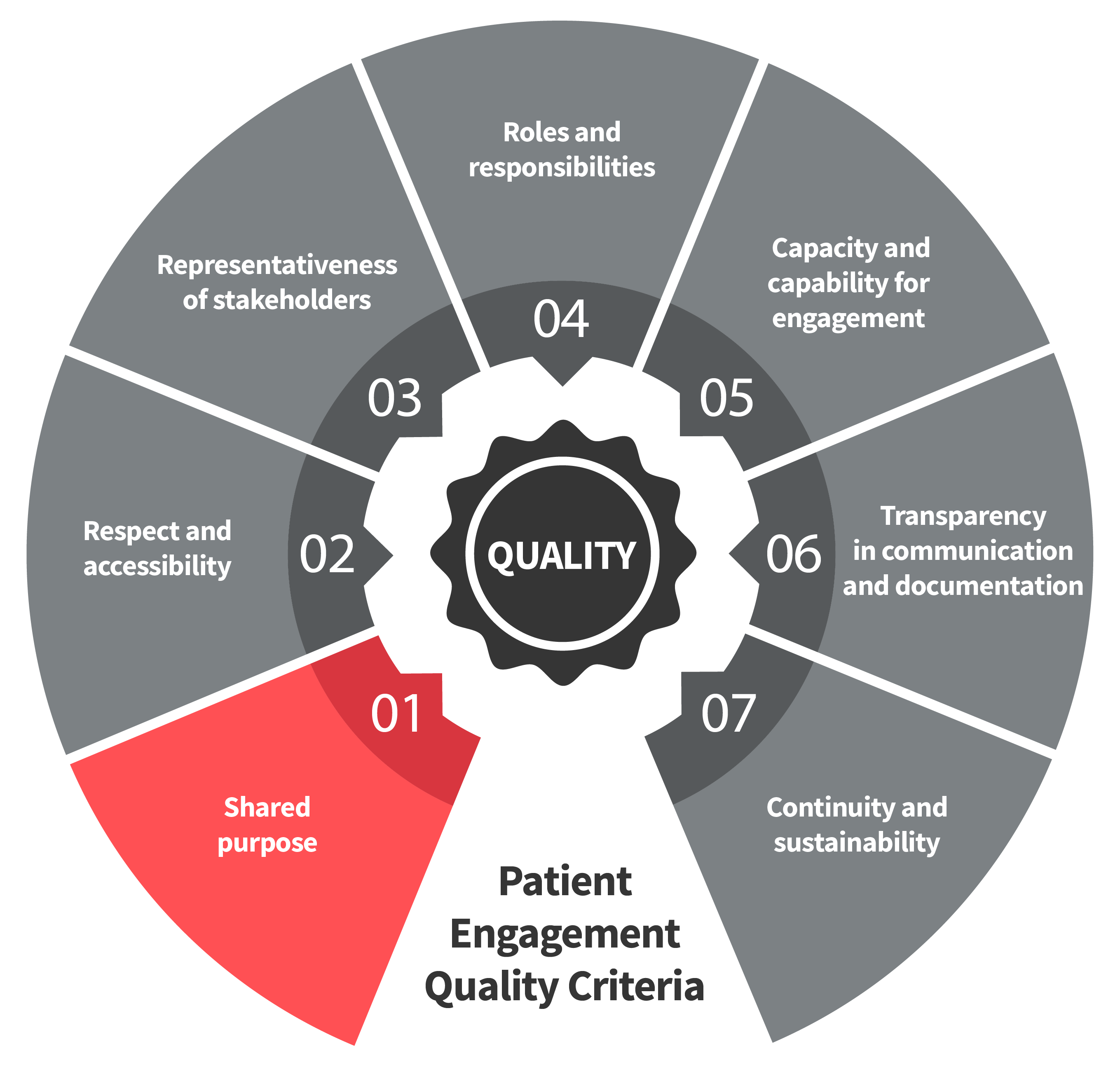
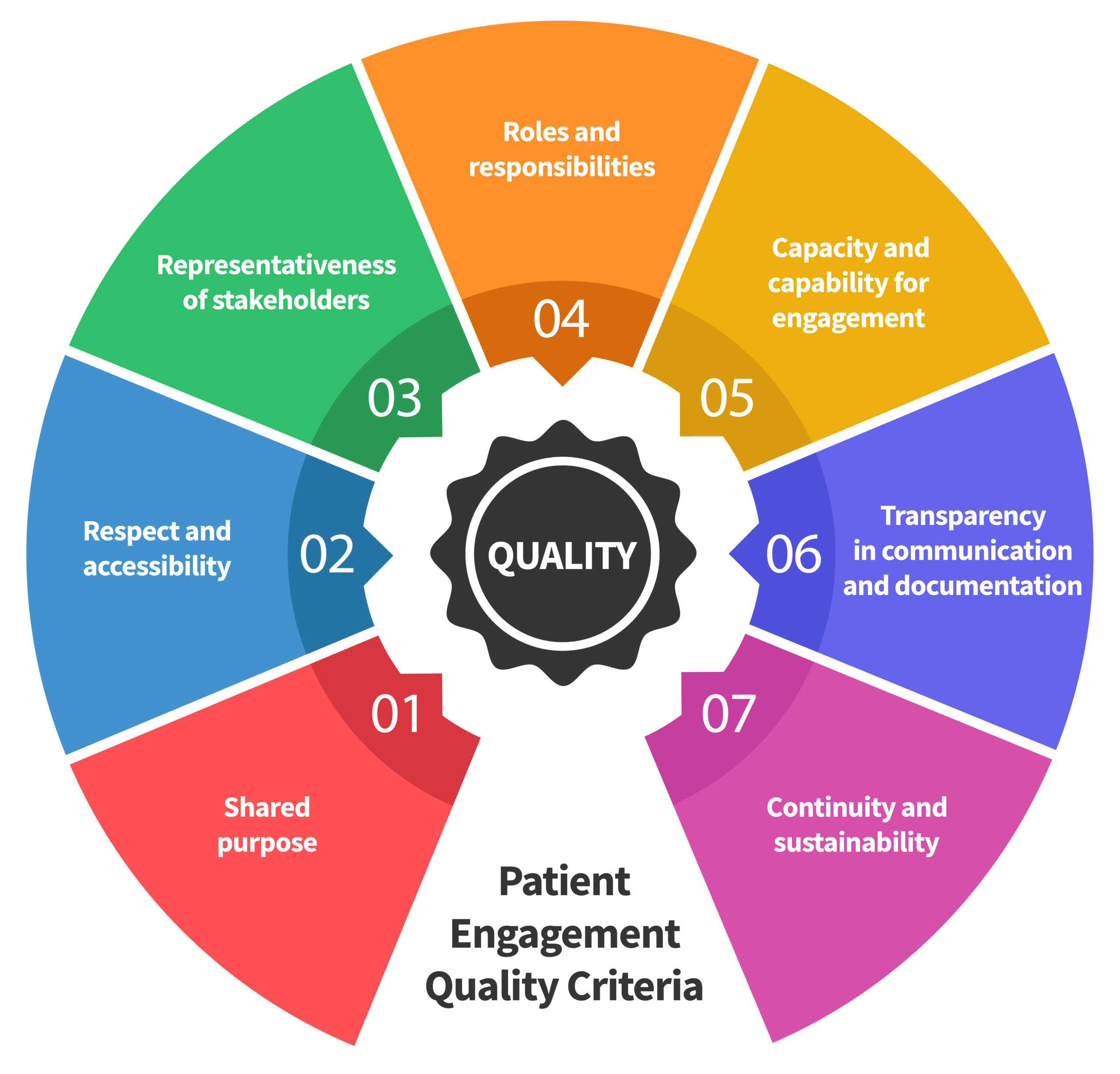
Seven Patient Engagement
Quality Criteria
The guidance introduces seven quality criteria to assess patient engagement practices. These have been consolidated from published PE frameworks and co-developed further by PFMD Contributors.
There are two versions or scenarios of the PE Quality Guidance Tool – scenario 1 will help you in planning a patient engagement project, and scenario 2 will help you assess your ongoing or completed project.

Who developed this tool
Subscribe to get the latest updates
The “Patient Engagement Management Suite” is brought to you by the PFMD partnership.